Diamond and Graphite both are known as the allotropes of carbon. The key difference between diamond graphite and fullerene is that diamond has a diamond cubic crystal structure and graphite has a hexagonal crystal structure while fullerene occurs as a large spheroidal molecule.

Different Allotropes Of Carbon Viz Graphite Diamond Fullerene And Carbon Nanotube Fullerene Graphite Carbon Nanotube
In diamond there is a three dimensional network of strong covalent bonds.
. The answer lies in the different atomic structures of diamond and graphite. Graphite is hard and has very little thermal heat capacityThis is a very high numberDuring the operation of electricity diamonds control electricity flows. Allotropy is therefore the existence of two or more different forms of an element in the same physical state.
These minerals chemically consist of carbon atoms with different physical properties. Diamond is vary hard whereas graphite is soft. Although they are made from the exact same component Carbon a Diamond differs from Graphite in the atomic structure.
However coke charcoal coal and lamp-black are amorphous forms of carbon. Which element has chemical properties that are most similar to the chemical properties of sodium. At STP graphite and diamond are two solid forms of carbon.
Graphite forms in layers or sheets where the carbon atoms have strong bonds on the same plane or layer but only weak bonds to the layer above or below. Because of the large gap between the molecules they have low density. Makes diamond useful for cutting tools such as diamond-tipped glass cutters and oil rig drills.
Diamond does not conduct electricity because it has no charged particles that are free to move. This makes diamond extremely hard. In these allotropes of carbon the atoms consisting of carbon atoms in that of the.
Diamond and graphite are both made entirely of carbon Why do they have radically different physical properties. - different crystalline lattice - different aspect - graphite is a good electrical conductor graphite not - diamond has a. Therefore as these allotropes are made up of same element so they have similar chemical properties but different physical properties like melting point boiling point etc.
Such different properties from two substances that are composed of exactly the same kinds of atoms. Diamond is one of the hardest materials known is transparent to light and does not conduct electricity at all. A sulfur stom has 6 valence electrons.
They have different atomic structures. Answer to Explain why the carbon materials of diamond Engineering. 3 Graphite is a metal but diamond is a nonmetal.
Because of hardness diamond is used in making cutting and grinding tools. This is the reason why Diamonds have a. Diamond graphite and fullerene are different allotropes of the chemical element carbon.
Since molecules are closely packed they have high density. 4 Graphite is a good conductor of. X and Y have atoms with the same number of outer shell electrons.
This is because carbon has the ability to exist as allotropes a phenomenon known as allotropy. They have different compositions b The carbon atoms in diamond are formed in earths core while the atoms in graphite are not c. Basically isotopes explain how diamond and graphite differ in different ways.
3The carbon atoms in graphite and the carbon atoms in diamond have different Aatoms have different atomic numbers Batoms have different atomic masses Cmolecules have different molecular structures Dmolecules have different average kinetic energies 4At 298 K oxygen O2 and ozone O3 have different properties because their. A diamond is a diamond with very little heat this is why the surface resembles diamond in its shape. These minerals in general are known to be as polymorphs having the same type of chemistry but of the various crystalline structures.
But this is not true at all conditions. The difference in the properties of diamond and graphite can be easily explained in terms their structures. Some differences between diamond and graphite.
It is soft and slippery and its hardness is less than one on the Mohs scale. Under extreme pressures diamond is more stable. Graphite is soft gray and can conduct electricity reasonably well.
Graphite does conduct electricity because it has delocalised electrons. On the periodic table the number of protons in an atom of an element is indicated by its. Which statement explains why these two forms of carbon differ in hardness.
Which statement explains why these two forms of carbon differ in hardness. Mechanical Engineering questions and answers. 2 Graphite and diamond have different molecular structures.
X and Y have different chemical properties. Which statement is correct. Graphite and diamond have different molecular structures.
And it is all due to how the carbon atoms are arranged. Graphite and diamond are the two crystalline forms of carbon. Which statement explains why sulfur is classified as a group 16 element.
Element X is a solid that is brittle lackluster and has 6 valence electrons. In graphite carbon atoms are joined together in sheets of six sided lattice. Diamond and Graphite have 2 different structures Diamond has a rigid tetrahedral network whereas Graphite has layers which completely changes the properties of both types of carbon.
Graphite and diamond have different. All these compounds have only carbon atoms in the. Graphites are formed due to the weak van der Waals force of attraction.
34 At STP graphite and diamond are two solid forms of carbon. In Diamonds the atoms are carefully packed with together with each atom connected to various other carbon atoms as compared to Graphite where the bonds in between the layers are weak. X and Y have atoms with different numbers of electron shells.
Diamond is hard and graphite is soft Match each physical property to the. In diamond strong three-dimensional networks are formed due to the presence of covalent bonds. Both graphite and diamond have very similar energies of formation at normal conditions though graphite is marginally more stable the particular arrangement of bonds has a slightly lower energy than the arrangement in diamond under normal conditions.
Whereas in diamond carbon atoms are joined together in four cornered lattice. 1 Graphite and diamond have different ionic radii. X and Y have atoms with the same nucleon number.
Unlike diamond graphite can be used as a lubricant or in pencils because the layers cleave readily. The carbon atoms in diamond on the other hand have strong bonds in three dimensions.

Why Diamond And Graphite Have Different Physical Properties But Same Chemical Properties What Is The Property Called Quora
Difference Between Diamond And Graphite Definition Properties Uses
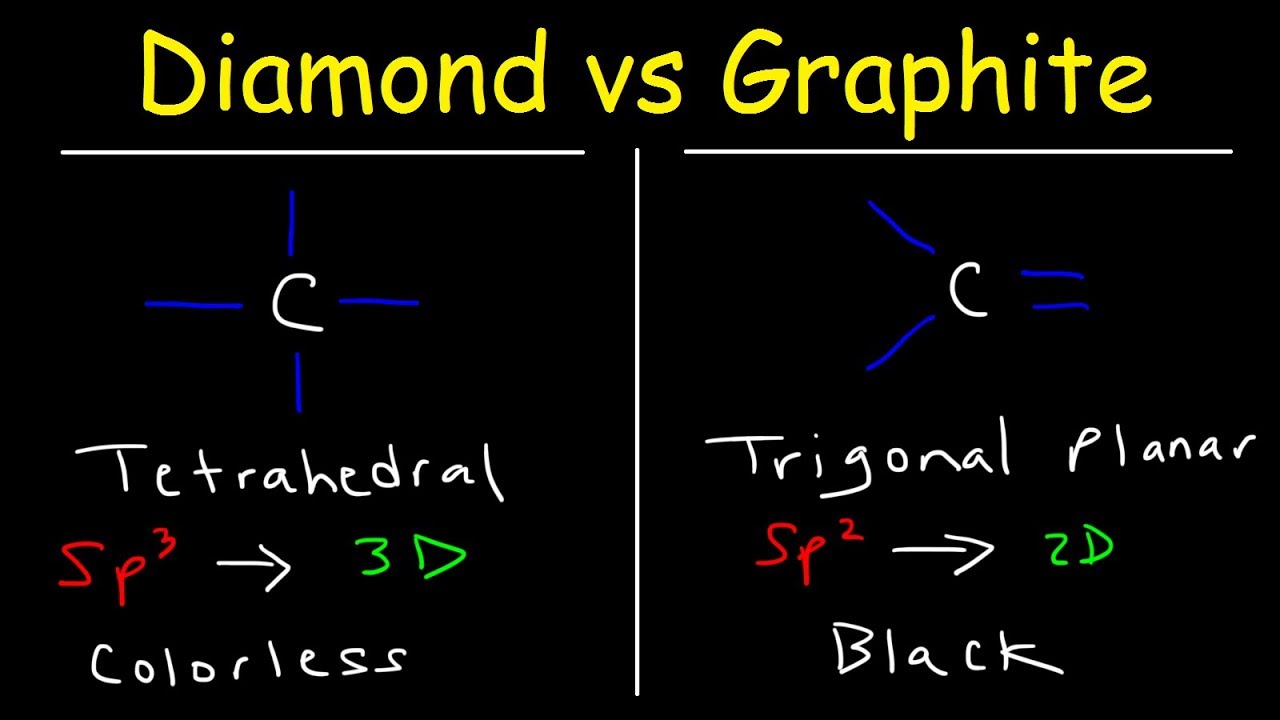
Structure Of Diamond And Graphite Properties Basic Introduction Youtube
0 Comments